views
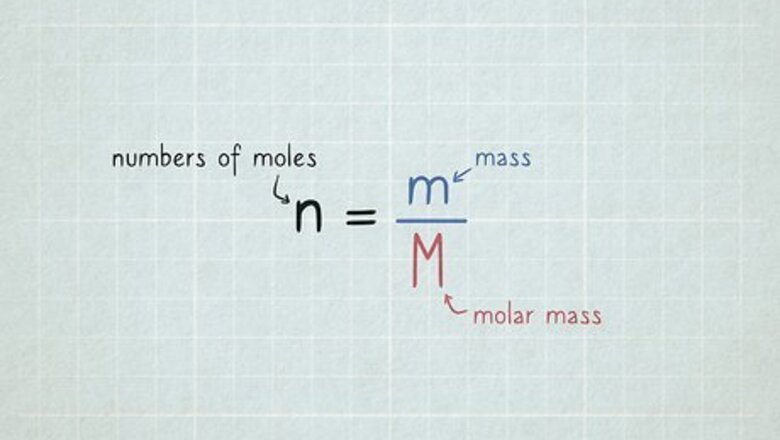
n
=
m
M
{\displaystyle n={\frac {m}{M}}}
and solve for
n
{\displaystyle n}
.
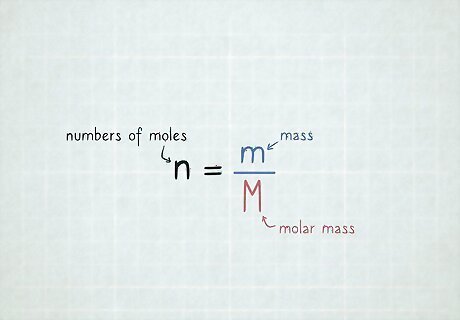
Set up your equation. The equation you'll need to use is n = m M {\displaystyle n={\frac {m}{M}}} n={\frac {m}{M}}, or the numbers of moles n {\displaystyle n} n is equal to the mass m {\displaystyle m} m divided by the molar mass M {\displaystyle M} M.

Substitute any given values. For example, if you have to calculate how many moles go into 74 g of hydrogen peroxide, you'll get n = 74 g 37 g m o l {\displaystyle n={\frac {74g}{37{\frac {g}{mol}}}}} n={\frac {74g}{37{\frac {g}{mol}}}}, since you have 74 g of hydrogen peroxide, and the molar mass is 37 g m o l {\displaystyle {\frac {g}{mol}}} {\frac {g}{mol}}.
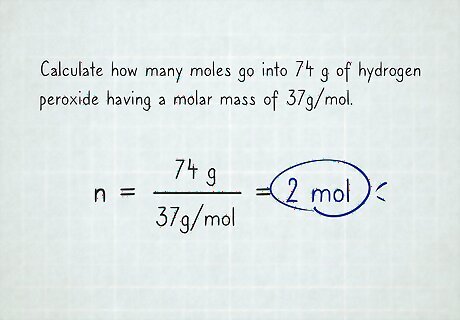
Solve the equation and cancel out the units. The unit grams is both in the numerator and denominator so that cancels out. Moles is in the denominator of the denominator, which leaves you with moles in the numerator. The final answer is n = 74 37 m o l = 2 m o l {\displaystyle n={\frac {74}{37}}mol=2mol} n={\frac {74}{37}}mol=2mol.
















Comments
0 comment