
views
Making a Sodium Acetate Solution

Pour vinegar in a pan. Vinegar is a dilute solution of acetic acid. The solution is primarily water, with anywhere between 3% and 7% acetic acid. The acetic acid is a necessary ingredient in forming sodium acetate. Pour 500 millilitres (2.1 c) of vinegar into a pan. Always wear safety goggles and gloves when handling acids and bases like vinegar and baking soda.

Add baking soda to the pan. Baking soda is the common name for sodium bicarbonate. This ingredient will provide the sodium needed to form sodium acetate. Slowly sprinkle about 35 grams (7 tsp) of baking soda for every 500 millilitres (2.1 c) of vinegar.

Stir the reaction. As you sprinkle the baking soda into the vinegar, you will see the solution start to bubble. This is due to the formation of carbon dioxide gas during the reaction. Use a stir rod or a spoon to keep the reaction moving, and prevent it from bubbling out of the container. The reaction of vinegar with baking soda is as follows: NaHCO3 + CH3COOH ---> CH3COONa + CO2 + H2O
Boiling Off Excess Water

Transfer the solution to a boiling pan. Any pan that is stove-safe will suffice. Transfer only the liquid solution. Do not pour solid baking soda into the boiling pan. You will only have solid baking soda if you added too much baking soda. The excess baking soda will remain in a solid (but wet) form.

Bring the solution to a boil. Put the boiling pan on the stove and bring the solution to a slow boil. Avoid boiling vigorously, as this will make it hard to monitor the surface of the solution, and could lead to over-boiling. You can also use a bunsen burner or hotplate to boil the solution.

Watch the surface of the solution. Be sure that the solution is at a light enough boil that you can monitor the surface of the solution. If it is boiling too vigorously for you to watch the surface, turn the heat down. Allow the solution to slow boil until you see a white solid start to form in the solution or on the surface. When you see this, remove from heat immediately and swirl the solution until the solid redissolves.

Allow the solution to cool. As the solution cools, the sodium acetate dissolved in the hot water will precipitate out. It may take up to half an hour before you notice the formation of white sodium acetate crystals. Once the crystals form, you can pour off any excess water. If the crystals do not form, the solution may be resting in a supersaturated state. This means that there is excess sodium acetate dissolved in the water for its current temperature. Drop a small piece of metal (even aluminum foil should work) into the solution to start the crystallization. If you are building hot ice sculptures, you will want to pour the solution a little at a time into your mold or design. This should catalyze the sodium acetate to precipitate out of solution, and form a solid sculpture.

Scrape the crystals off. The crystals will form on the surface of the dish. For the best yield, scrape them off with a razor. Collect the crystals in an airtight container (a ziplock bag is sufficient). If you plan to make a hand warmer, put the crystals in an airtight plastic bag. You can melt the crystals by dropping the bag into boiling water. Leave it in liquid form until you need a hand warmer, then drop a crystal or piece of metal in to catalyze the change back to a warm solid.
Evaporating Excess Water

Pour the solution into an evaporating dish. An evaporating dish will allow the water and carbon dioxide to slowly evaporate from the crystals. This method will take much longer than boiling off excess water and carbon dioxide, but can be done. Do not transfer any solid baking soda particles into the evaporating dish. A wide/long, shallow dish, like a glass casserole dish, works best. The water will take much longer to evaporate from a deep dish.
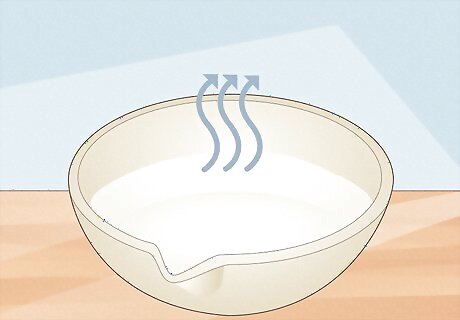
Allow the contaminants to evaporate. The evaporation process will take days under standard conditions (room temperature, normal atmospheric pressure, etc.). If you wish to speed up the evaporation, you can place the dish under a heat lamp. As the water evaporates, white sodium acetate crystals will precipitate out of the solution and cling to the dish.

Collect the crystals. Once the water evaporates, the sodium acetate crystals will be stuck to the evaporating dish. Use a razor to scrape the crystals from the dish. Store the crystals in an airtight container such as a ziplock bag.















Comments
0 comment