
views
The very nascent bond of atoms, which occurred at the onset of the universe 13 billion years ago, has been discovered in the astrophysical nebulae for the very first time. The latest observation of helium hydride (HeH+) marks a fascinating point in the realms of science and technology, giving us a peek at the natural bond formation, which is essentially the foundation block of the formation of the universe.
The formation of HeH+ marks the beginning of chemistry as well as the universe, occurring subsequently in time just after the first nucleosynthesis, more commonly referred to as the Big Bang. At this time, the temperature of the universe, which was still at its nascent stage, fell below 3,726°C, leading to the nucleon clusters of hydrogen, helium, deuterium and lithium (in traces) to re-combine in descending order of their ionisation potential (ions with the highest ionisation potential recombined first). This led to the formation of helium, which combined with free electrons to form a neutral atom — the first one ever.
At this stage, this helium atom subsequently combined with the still-ionised hydrogen atom (with helium higher in ionisation energy) to create what we finally discovered a few days ago — helium hydride, or HeH+. It is this molecular bond that led to the eventual formation of molecular hydrogen, which is the foundation module of the world’s inception. This astrophysical observation, made by researchers using the GREAT spectrometer on board the SOFIA observatory in low orbit, marks a pivotal point of mankind’s understanding of the starting point of the universe, and how the world’s first chemical reaction led to the complex interweb of chemical bond formations, that hold together the world as we know it today.
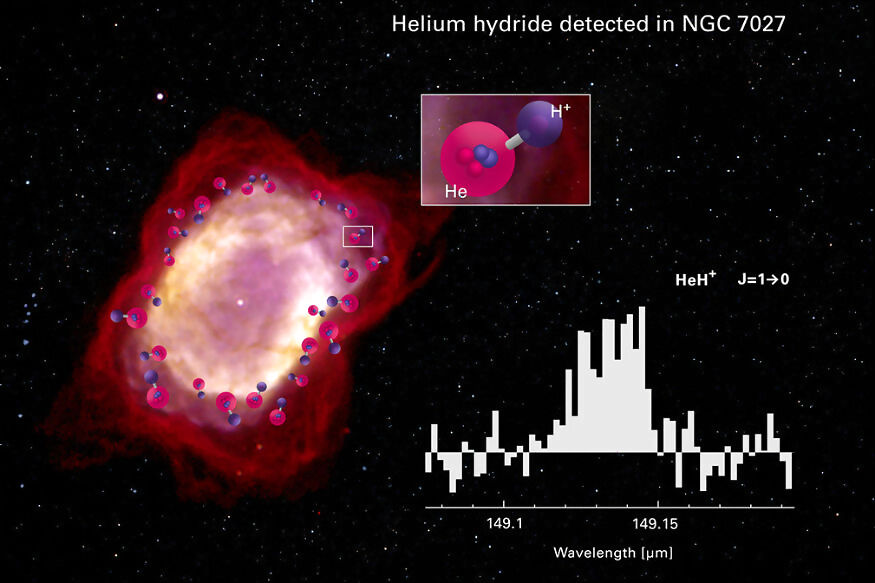
Interestingly, helium hydride was first observed on Earth, in laboratory conditions, as early as 1925. Ever since then, repeated attempts and research projects into observing the HeH+ bond formation in nature has proved elusive. It was believed to exist in abundance in planetary nebulae, which is ejected in a field envelope at the last stage of a star. During this, the rising temperatures lead to driving ionisation fronts, where HeH+ is seemingly formed. While the observation in theory should have been straightforward, it took great technological advances in high resolution spectroscopy, which made it possible for scientists to make observations at the requisite, far-infrared wavelength.
David Neufeld, who co-authored the paper, stated, “The discovery of HeH+ is a dramatic and beautiful demonstration of Nature's tendency to form molecules. Despite the unpromising ingredients that are available, a mixture of hydrogen with the unreactive noble gas helium, and a harsh environment at thousands of degrees Celsius, a fragile molecule forms. Remarkably, this phenomenon can not only be observed by astronomers but also understood using theoretical models that we have developed.”
The discovery of the world’s first ever molecular bond is not only mesmerising, but comes right on the heels of humans capturing their first glimpse of a black hole, and possibly fathoming the presence of the first ever recorded interstellar object on Earth. This does not simply mark a momentous occasion in the field of astronomy, but opens far wider boundaries in terms of astronomical discoveries and research programmes, in years to come.




















Comments
0 comment