
views
Obtaining Titanium Dioxide

Collect donut powder. Buy a bag of white powdered donuts. The powder contains a chemical called titanium dioxide (TiO2). Titanium dioxide is a useful material for creating solar cells.
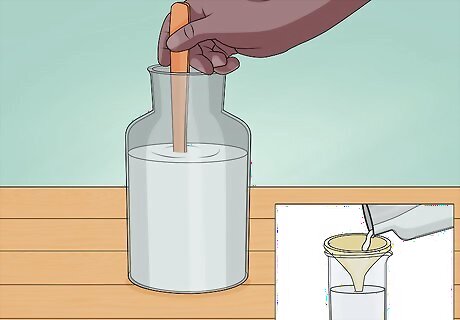
Dissolve the sugar. Unfortunately, the titanium dioxide you get from a powdered donut will be not be pure. It will be mixed with sugars and fats. To remove the sugar, stir the powder into warm water and then pour it through a filter (a coffee filter will work fine). The sugar will dissolve in the water and pass right through the filter. The solid left behind is a mixture of titanium dioxide and fats. Use roughly one cup of water for every five donuts.

Remove the fat. The fats are not water soluble, and so they remain mixed with the titanium dioxide after filtration. Fortunately, it is quite easy to remove them. Put the powder on a heat safe dish and bake it at 500 °F (260 °C) for about three hours. This will vaporize the fats and leave behind a titanium dioxide powder.
Creating a Solar Cell
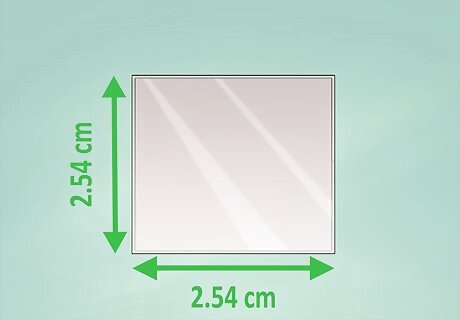
Use conductive glass. Most conductive glasses are coated with an indium tin oxide residue. This allows the surface of the glass to conduct electricity rather than insulate it. You can buy conductive glass online or at a solar supply store. This glass is usually found in 1x1in (2.54x2.54 cm) squares.
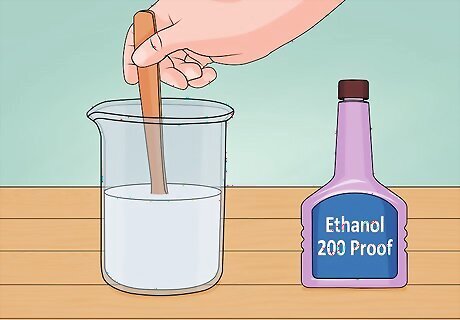
Make a titanium dioxide solution. Add ethanol to your titanium dioxide in a glass beaker and stir. You should use the most pure ethanol you can find. Two hundred proof lab grade ethanol is best, but vodka or Everclear will work in a pinch. Use about one milliliter of ethanol per donut and shake or stir the solution in a glass or beaker.
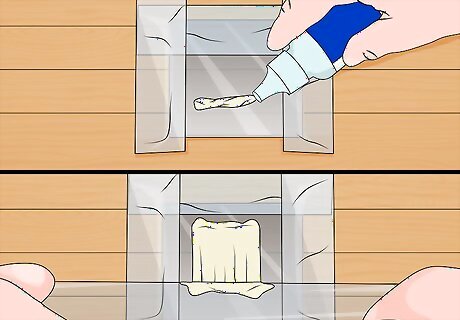
Coat the glass. Put a layer of tape around three sides of the glass. This will help you control the depth of your coating. Use a dropper or pipet to drop a small amount of the titanium dioxide solution onto the surface of the glass. Use a microscope slide to scrape the the excess liquid off, leaving just a thin coating. Repeat this process ten times. Each drop should be sufficient to coat the glass one time with a thin film. In total, you will use ten drops to form ten layers of titanium dioxide.

Cook the solar cell. Put the solar cell into a clear, heatproof beaker or dish. Place the container on a hotplate (or place the solar cell directly on the hotplate). Turn the hotplate on and cook the cell for 10-20 minutes. You will have to watch the cell closely. It will turn brown, and then back to white. When the cell goes back to its original white color, this means that the organic solvents (the ethanol) have burned off, and the cell is finished heating.
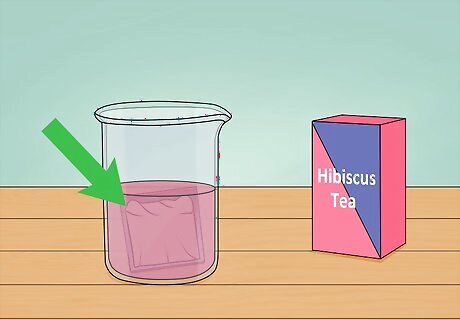
Stain the cells with tea. Teas contain organic compounds known as anthocyanins. These compounds are good at capturing light in the visible spectrum. Brew a cup of herbal tea and submerge the solar cell for a few hours. Darker teas, such as hibiscus, work best. This will stain the cell and allow anthocyanins to bind to the surface of the cell. The cell is now capable of capturing visible light. Prior to staining, the cell could only capture light in the UV spectrum.
Generating a Current
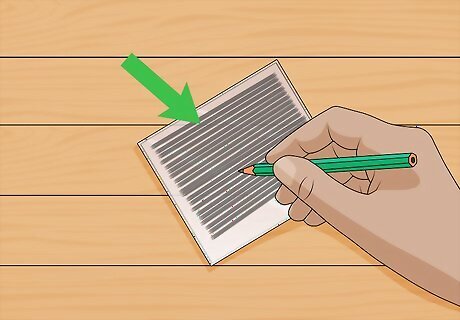
Color another piece of conductive glass with graphite. This peice of glass will act as a counter-electrode. You can use a regular graphite pencil. Just rub the tip of the pencil over the glass until it is fully covered with the graphite residue.
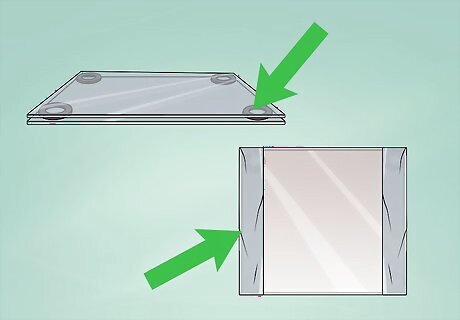
Put a spacer between pieces of glass. You can cut a thin plastic spacer to put between the pieces of glass. The spacer should go on the clean side of the glass (opposite the tea or graphite side). Alternatively, you can put tape around the edges on the clean side of the glass to form a spacer. This keeps the glass ever so slightly separated.
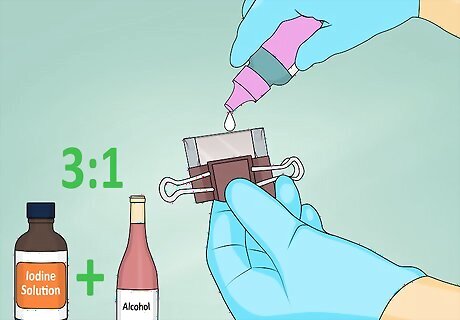
Add an electrolyte solution. An iodine solution is an ideal electrolyte. You can get it over the counter at most pharmacies. Mix the solution in a 3:1 ratio with alcohol. Put one to two drops of the solution between the two pieces of glass.
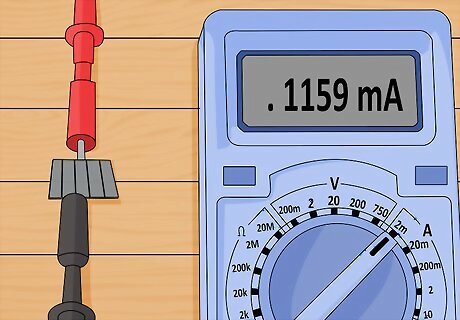
Press the pieces of glass together. Before the solution has time to evaporate, press the two pieces of glass firmly together. Use alligator clips to hold them in place. Your cell is now capable of producing electrical currents when exposed to light. You can test this by placing the cell in sunlight and using a multimeter to check for current.
















Comments
0 comment