views
Reading a Barometer
Look for the trend. In gauging weather trends and analysis, the absolute value of the pressure is nowhere nearly as significant as its "trend." Namely: is the barometric pressure rising, is it falling, or is it holding steady? Watch the barometer needle and record its movements. The dials of older barometers often feature artistically-drawn backgrounds to indicate weather conditions like storms, strong winds, and clear skies. For all their aesthetic, these backgrounds can be misleading. The movement of the barometer needle has far more bearing upon the coming weather. If you have an old, traditional mercury tube-type barometer, then you may need to watch the meniscus: the highest curve of the liquid mercury that fills the cylinder.
Look at the reading. To determine the trend in a barometer, you need to compare the current pressure reading to a past pressure reading. Calculate the difference in pressure between the current reading and the reading from an hour ago. In many barometers, you can manually set a needle to mark a point on the pressure gauge. The needle will remain at this point to help you gauge recent trends in the pressure.
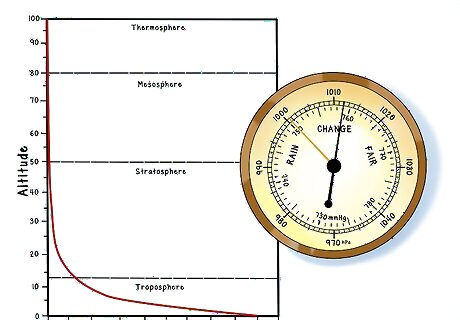
Know that atmospheric pressure decreases more or less exponentially with altitude. The higher you go, the lower the pressure. This means that a barometric pressure that would send someone straight through the trapdoors of a storm cellar at sea level along the coast of Costa Rica would be perfectly commonplace in the midst of summer in the mile-high city of Denver.
Calculating Pressure
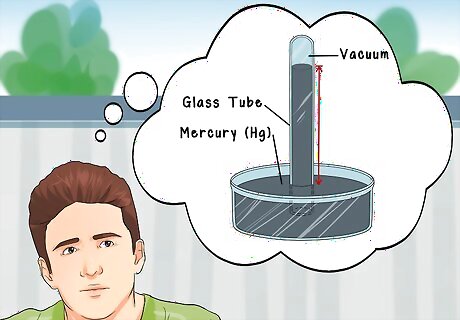
Understand the origins of barometric measurement. The Italian physicist Torricelli conceived the first barometer from the fact that the average pressure of the atmosphere is capable of "sucking" 76 centimeters (760 mm) of mercury (Hg, which is a liquid metal at STP) up the inside bore of an evacuated glass tube. Later mathematicians have come up with other units of pressure, but the traditional unit remains mm Hg: millimeters of mercury.

Know the units of pressure. Pressure is a measure of force per unit area, and there are various ways to describe both the force and the area. Atmospheric pressure is usually expressed in psi (pounds per square inch). It can also be expressed in "atmospheres" – one atmosphere is 14.7 psi. In the U.S., it is common to speak of air pressure in "inches of mercury," as one might read the pressure from a barometer. In meteorology, air pressure is most often expressed in "millibars:" each millibar is exactly one dyne (gm-cm/sec^2) per square centimeter in the c.g.s. system of units. 14.7 psi is a rough average of barometric pressure at sea level and at STP (standard temperature and pressure.) STP is an internationally-accepted "general state" of the atmosphere. The 14.7 figure has been averaged over a large number of measurements, all either taken at sea level or corrected to sea level. Atmospheres are rarely used in meteorology. The millibar is an especially convenient unit of pressure for atmospheric studies. 1033 millibars is the pressure equivalent to one atmosphere, 14.7 psi, or 30 inches of mercury. Most weather maps and all aeronautical weather charts are in millibars, and the pressure at sea level is usually very close to 1000 millibars. Nearly all barometers in the U.S. are graduated in units of inches of mercury. The barometer is read to the nearest hundredth of an inch, such as "29.93 inches." Similarly, aircraft altimeter settings are universally given by control towers in inches of mercury corrected to sea level, regardless of the altitude of the airfield.
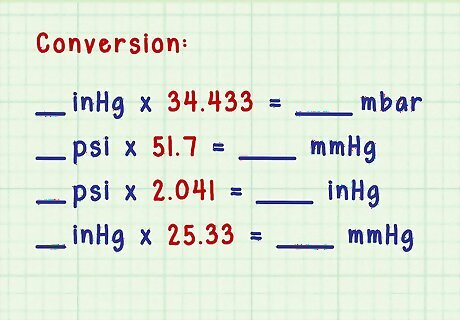
Convert between units of pressure. If you’re simply converting a measurement from one unit of pressure to another, you can learn the multipliers to convert between millibars, psi, atmospheres, and mm of mercury. Convert from inches of mercury (read from the barometer) to millibars: if you know the inches of mercury, simply multiply by 34.433. 1033 / 30 = 34.433 {\displaystyle 1033/30=34.433} 1033/30=34.433 Convert from psi to mm of mercury: multiply the psi by 51.7. 760 / 14.7 = 51.7 {\displaystyle 760/14.7=51.7} 760/14.7=51.7 Convert from psi to inches of mercury: multiply the psi measurement by 2.041. 30 / 14.7 = 2.041 {\displaystyle 30/14.7=2.041} 30/14.7=2.041 Convert from inches of Hg (mercury) to mm Hg: multiply the measurement in inches by 25.33. 760 / 30 = 25.33 {\displaystyle 760/30=25.33} 760/30=25.33

















Comments
0 comment