views
X
Research source
In order to calculate the molecular weight of a formula, you'll need to add up the atomic masses of each element present.
Calculating Molecular Weight
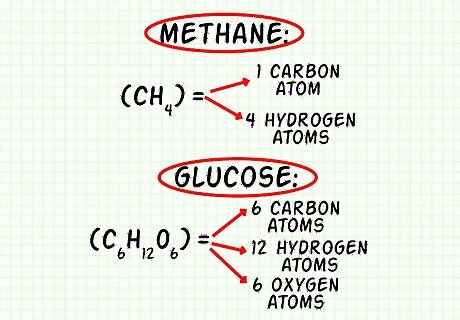
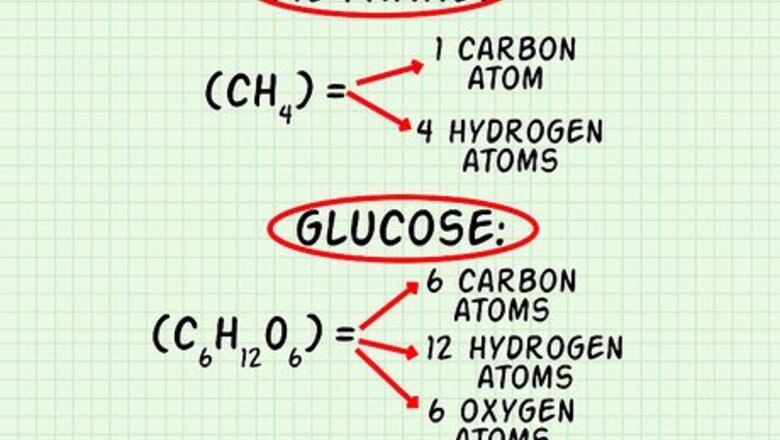
Count how many atoms of each element exist in the molecule. First, list each element present in the molecule. You may use the chemical symbol or write out the name of the element. Then, count the atoms according to the molecular formula and write them next to the element’s name or symbol. For example, carbon dioxide or CO2, list that there is 1 carbon (C) and 2 oxygens (O) present in the molecule. For methane, CH4, list carbon (C) and hydrogen (H). Methane comprises 1 atom of carbon and 4 atoms of hydrogen. For glucose, C6H12O6, list carbon (C), hydrogen (H), and oxygen (O). Glucose comprises 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen.
Find the relative atomic mass of each element in the molecule. Use a copy of the Periodic Table of Elements. The Periodic Table lists the atomic mass of each element below the chemical symbol. For example, oxygen has a relative atomic mass of 15.9994 amu. For carbon dioxide (CO2), the relative atomic mass is 12.011 amu for carbon and 15.999 for oxygen. The atomic mass of an element is roughly equal to the summed mass of the protons and neutrons that it contains. Note that the relative atomic mass you find in the periodic table is scaled: it accounts for all of the isotopes of the element in the proportions that they naturally occur.
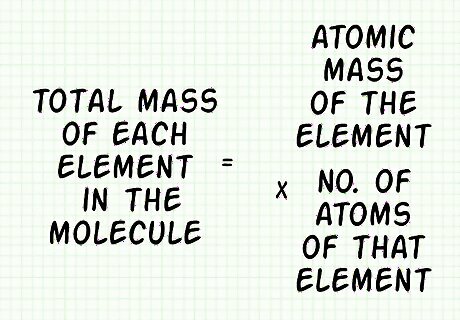
Calculate the total mass for each element in the molecule. Multiply the atomic mass of each element by the number of atoms of that element: (Atomic Mass of Element) x (# of atoms of that element). Do this for each element in the molecule. In our carbon dioxide example, the mass of the single carbon atom is 12.011 amu. Since there are 2 oxygen atoms, you would write 15.999 ∗ 2 = 31.998 a m u {\displaystyle 15.999*2=31.998amu} 15.999*2=31.998amu.
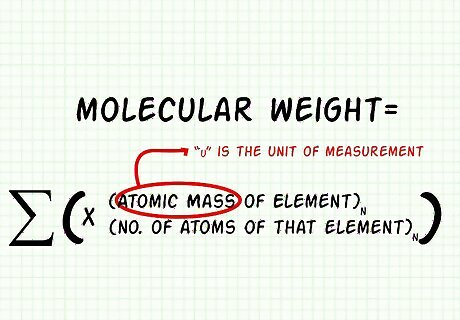
Add up the mass of all the atoms to find the molecular weight. Molecular weight = ∑((Atomic Mass of Element)n x (# of atoms of that element)n) Round the answer as necessary, using significant digits. Remember to use the proper units. amu is the old abbreviation for atomic mass units, but the "most correct" modern unit is a lower-case u. For carbon dioxide, the molecular weight can be found like so: 12.011 + 31.998 = 44.009 u {\displaystyle 12.011+31.998=44.009u} 12.011+31.998=44.009u.
Practicing with Examples
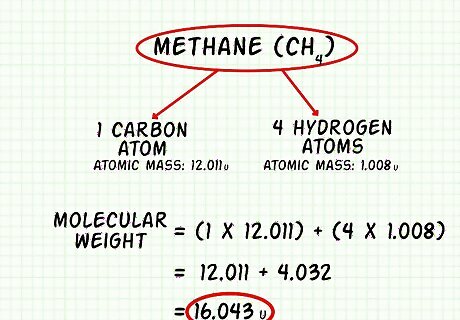
Find the molecular weight of methane (CH4). The atomic mass of hydrogen is 1.008 u. The atomic mass of carbon is 12.011 u. There are 4 hydrogen atoms in methane. Add together the weight of all the atoms in methane to find the molecular weight. 1.008 ∗ 4 = 4.032 u {\displaystyle 1.008*4=4.032u} 1.008*4=4.032u. 12.011 ∗ 1 = 12.011 u {\displaystyle 12.011*1=12.011u} 12.011*1=12.011u 12.011 + 4.032 = 16.043 u {\displaystyle 12.011+4.032=16.043u} 12.011+4.032=16.043u
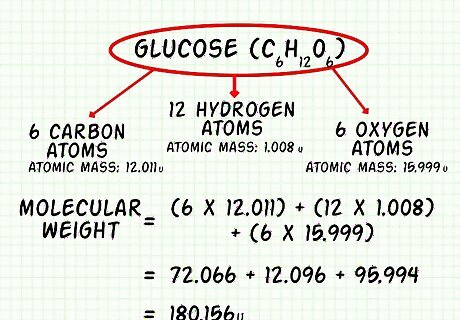
Calculate the molecular weight of glucose (C6H12O6). The atomic mass of carbon is 12.011 u. The atomic mass of hydrogen is 1.008 u. The atomic mass of oxygen is 15.999 There are 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. Add up the mass of all of the atoms in glucose to calculate the molecular weight. 12.011 ∗ 6 = 72.066 u {\displaystyle 12.011*6=72.066u} 12.011*6=72.066u 1 , 008 ∗ 12 = 12.096 u {\displaystyle 1,008*12=12.096u} 1,008*12=12.096u 15.999 ∗ 6 = 95.994 u {\displaystyle 15.999*6=95.994u} 15.999*6=95.994u 72.066 + 12.096 + 95.994 = 180.156 u {\displaystyle 72.066+12.096+95.994=180.156u} 72.066+12.096+95.994=180.156u


















Comments
0 comment