views
Mumbai: The Indian Patent Office has given the Hyderabad-based Natco Pharma Limited the go-ahead in the form of a compulsory license to legally make and sell a low-cost generic version of German company Bayer's anti-cancer drug, Nexavar.
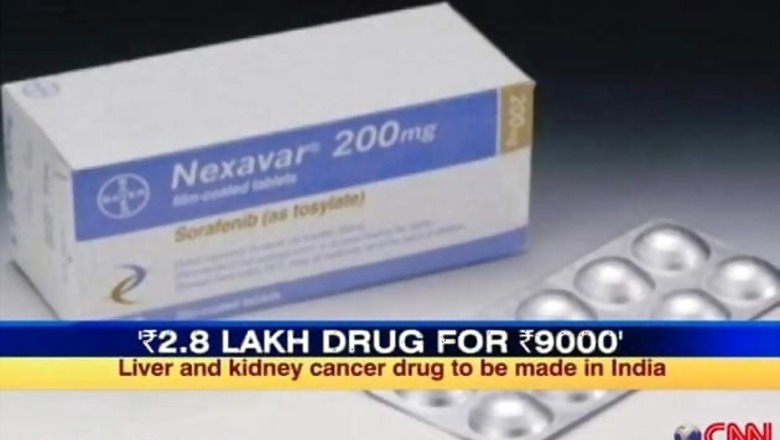
The Controller of Patents PH Kurian, in a 62-page ruling, observed that with Nexavar priced at Rs 2,80,000 for a month's treatment, Bayer has not adequately worked the patent, adding that Nexavar was made available to only 8842 patients, only two per cent of eligible patient population.
Nexavar is used to treat end-stage cancer - stage four cancers - giving patients, a few months reprieve. A patient that CNN-IBN spoke to and who is too sick to come on camera is already on Natco's generic drugs. Oncologists say there are very few Indian cancer patients who can afford the Rs 2.8 lakh Bayer drug.
The landmark ruling will see Natco legally selling a copy of the anti-cancer drug, at Rs 8,800 for a month's treatment, while paying six per cent royalty to Bayer.
"It's a bold decision by the patent controller and we are very proud of it. We will be selling the monthly medication of 120 tablets at Rs 8,880, instead of Bayer's Rs 2.8 lakh. It should hit the market in 15-30 days," said M Adinarayana, Company Secretary and General Manager, Corporate and Legal Affairs, Natco.
While this has far-reaching implications, even for other drugs, the decision will not go undisputed.
"It is a big move and could have ramifications for other drugs. But this decision will lead to conflict," said pharma analyst Vikas Dandekar.
Bayer has already reacted, saying, "We are disappointed by the decision of the Patent Controller in India to grant a compulsory license for Nexavar. We will evaluate our options to further defend our intellectual property rights in India."













Comments
0 comment