views
Covaxin was assessed on “exactly the same” criteria as other vaccines, the World Health Organisation told News18.com, refuting the claims of Bharat Biotech’s chief Dr Krishna Ella who had questioned the “excessive” scrutiny.
In an emailed response to the queries sent by News18.com, WHO replied that “The emergency use listing process is a neutral, technically rigorous process and non-political, with independent regulatory experts contributing to evaluations and advising WHO.”
Moreover, WHO added that “Covaxin was assessed according to the exact same criteria as other vaccines.”
Speaking at an event, Dr. Ella, chairman of the Hyderabad-based vaccine firm, had on Wednesday said: “We are the only vaccine in the WHO that has gone through so much scrutiny which other vaccines have not gone through. But it’s good that in the end, we won the game.”
Dr Ella further said that it was not about the process but the WHO might have got influenced by a lot of what was said by negativists in the country about the vaccine. He also said the criticism that it was a “Modi vaccine” also got the process delayed.
“They (WHO) wanted to be doubly sure of what they were doing. So, they wanted to review much more intensely. Every small issue became a major issue for them.”
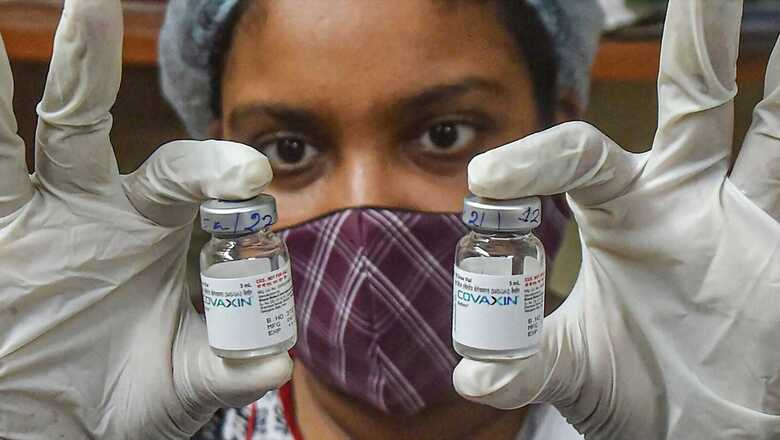
After much back and forth, the WHO granted emergency use listing (EUL) to Covaxin, adding to a growing portfolio of vaccines validated by the global health body for the prevention of Covid-19.
Covaxin is a whole virion-inactivated vaccine against SARS-CoV2, developed in partnership with ICMR and NIV, Pune, Bharat Biotech said in a statement. With validation from the World Health Organisation (WHO), countries can now expedite their regulatory approval processes to import and administer Covaxin, it added.
United Nations Children’s Fund (UNICEF), Pan-American Health Organisation (PAHO) and GAVI COVAX facility will also be able to procure Covaxin for distribution to countries worldwide, Bharat Biotech said.
“Validation by WHO is a very significant step towards ensuring global access to India’s widely administered, safe, and efficacious Covaxin,” Ella had said.
Read all the Latest India News here

















Comments
0 comment