views
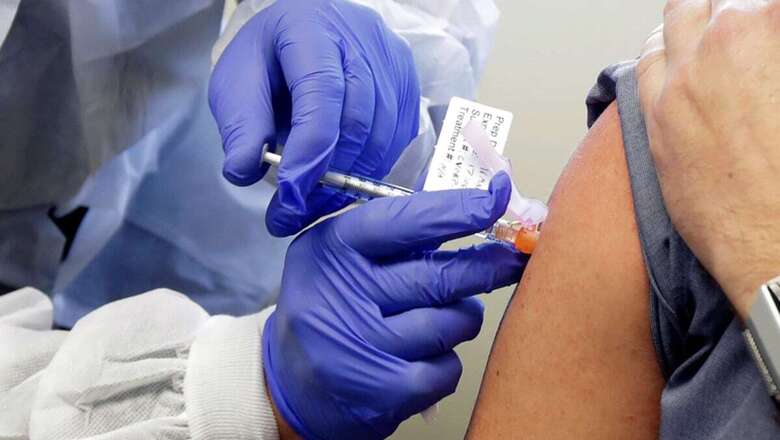
Top scientist and Department of Biotechnology Secretary Dr Renu Swarup has been actively involved in delivering a vaccine against Covid-19 to India. A scientist and a bureaucrat, her department under the Ministry of Science and Technology has been at the forefront of aiding the vaccine research. Speaking to News18, Swarup elaborates on her vision for vaccine development initiatives, and on how India will not just develop a vaccine for itself but also for its neighbours.
Edited excerpts:
The government has announced Rs 900 crore to develop a vaccine against Covid-19. How will this money be spent?
This is primarily for the Covid Suraksha mission. The mission has a very clear mandate and that is how we are going to accelerate the pace of development of our indigenous vaccines and get them to market as quickly as possible. Creating an ecosystem for this is the whole purpose. We are working with all our vaccine developers to see how we can help accelerate this through Covid Suraksha mission.
This was a much needed move given how less we were spending in R&D…
We have been able to see success because of the sound foundation that we were able to create. This foundation has been through the concerted effort to build an ecosystem. We have the National Bio Pharma Mission. We created shared infrastructure, built capacities for start-ups, for research groups. We also partnered with CEPI, one of the key players now in vaccine development. We launched an IND CEPI mission.
At that point we didn’t know it would be for a pandemic but you see how quickly we responded. The ecosystem responded. We also have to recognize that we have the best vaccine developers and manufacturers in our country. The credibility our vaccine developers have is such that more than 60% of the global immunization programme is through the indigenous vaccines that are developed and manufactured here.
Is it true that you can develop 5-6 vaccines with this money? Is all of it for Covid-19 or is there something else you are looking at?
Yes, our mandate is to develop at least 5-6 vaccines, all for Covid-19. This is under the Covid-19 vaccine mission. As I said, this Rs 900 crore has not only come for the vaccines, but this is along with the funding we have been given to bring the vaccine up to the level of pre-clinical trials. Once they have reached the pre-clinical trials and start human trials, we have to go for manufacturing, build capacities in the clinical trial sites in the immunogenicity assay labs, in being able to create facilities of shared infrastructure in which we can bring out animal challenge models. The whole purpose is how do we create a robust ecosystem. This will definitely go beyond Covid. Right now the effort is to deliver for Covid-19 in an accelerated pace.
How many vaccines has the Department of Biotechnology supported? You have funded India’s first mRNA vaccine. Tell us about that.
We have 30 vaccine candidates in the pipeline and they are at different stages. These include vaccines in research in academic institutes, some start-ups are doing research as well. We have supported many of them. Support is not always in terms of direct funding.
Making available the technology, the guidance, the advice, connecting them to right groups and facilitating them is the kind of support we offer. We have four of our indigenous vaccines in human trials phase. We have others which are waiting to get into human trials. We spoke about the mRNA vaccine which we are supporting. They have put in an application for clearance for human trials. The fact that they could complete pre-clinical studies and put an application for human trials is very encouraging.
mRNA is a new technology, a new platform, we have a lot of hope in this technology. What is also interesting is that the particular technology being developed by Gennova Biopharmaceuticals will be a vaccine available at 2-8 degree Celsius.
You mean an mRNA vaccine suited to temperatures that suit us?
Yes. We will wait for data however. But that is being developed. Scientists are always very optimistic about the research we do.
Tell us a little about Zydus Cadila.
Yes, we are also supporting under the DBT-BIRAC consortium. We do have Zydus Cadila as a candidate. They are working with THSTI which is our institute to do the studies for the vaccine development. Again this is a novel technology. The DNA technology is being used. This has a lot of merit. Both DNA and mRNA technology help manufacturing become so much simpler. We are hoping both will be very effective.
Will the chain of transmission be broken if most people do not get the vaccine?
All this is being discussed right now. We have to understand that this is a new virus. There is still a lot of knowledge generation which is happening even now. It is hardly a period of 10 months. We are still trying to understand the infection part of it. Clinicians are working on it to understand when herd immunity will come, what is the size of population that needs to be immunised first. The government has put out its priority list, we need to focus now. I expect the information on all this to become clearer as time progresses.
How many vaccines will India have by early next year?
We have eight vaccines in the early stages of development. How we roll out will be on the basis of data. Some are in advanced stages. It will be a continuous movement now. They are all at a stage where they have done pre-clinical studies. They will keep rolling out. Now there are eight, we will have more. We are hopeful.
Please explain how efficacy studies will be ongoing even after the approvals.
We have adaptive trials of Covid-19 vaccine. We have never had a situation where for one particular target we have so many vaccines. Data as it comes will guide the trials. This will be continuously developed.
India will be the vaccine hub with Nepal, Bangladesh and Sri Lanka. Are you going to do trials in any of these countries?
The Prime Minister made it clear that India will make vaccines not just for itself, but also the globe. The intention is to work with our neighbouring countries and we are doing it. We have created PACT — partnerships for accelerated clinical trials — and in this we have started a training programme where we are trying to build capacities for clinical trials in these countries.
We had a couple of sessions. We brought in experts to make them understand what are clinical trials and the training for it. That’s one engagement. Second is where we tried to discuss clinical trials of our own vaccines. So discussions are on. The key aspect is how we could make vaccines from India available to other countries as well. There is a discussion with WHO and we are considering putting up a solidarity trial which may happen. But if that happens, we will have global vaccines to be tested at common sites.
Read all the Latest News, Breaking News and Coronavirus News here
















Comments
0 comment