views
Using an Electrical Conductivity Meter
Gather your materials. Before attempting to measure the TDS of your sample, make sure to prepare a clean, clear space with the appropriate instruments and tools for the task. If you do not have access to the materials necessary for this procedure, they can all be purchased easily online. You will need the following: A clean, properly sterilized beaker that is free of dust or other particles A sample of the water you want to analyze, collected into the sterilized beaker. Ideally, the sample should be at 25° C (or 77° F) at the time of analysis. An electrical conductivity meter — a device used to measure a solution's ability to conduct electricity. It works by releasing a current into a liquid, then measuring the resistance.
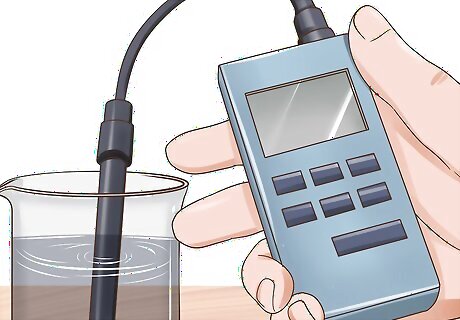
Measure the conductivity of the sample. Make sure your beaker with the water sample in it is placed on a flat, stable surface. Turn on the electrical conductivity meter, then insert the measuring lead into the sample. Wait for the reading on the conductivity meter to become stable before noting the result. You may have to wait a few seconds before the reading stabilizes, but it's important that you wait until the number on the display stops changing. The measurement displayed on the electrical conductivity meter is the purity of the water, measured in µS (micro-Siemens). The lower the µS value, the purer the water, with 0 µS being pure, unpolluted H20.
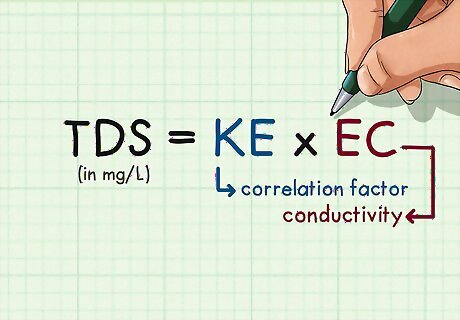
Plug your data into the TDS formula. The basic formula for calculating total dissolved solids looks like the above illustration. In the formula, TDS is measured in mg/L, EC is the conductivity of your sample (the reading from your electrical conductivity meter), and ke is the correlation factor. The correlation factor depends on the liquid being used as the sample, and it may also vary according to atmospheric conditions. It varies between 0.55 and 0.8. In the example above, say the correlation factor at the current temperature and in the current pressure conditions is 0.67. Plug your values into the formula. The TDS for your sample is therefore 288.1 mg/L. Water with a TDS of less than 500 mg/L meets the Environmental Protection Agency's standards for drinking water. A high TDS does not necessarily mean that water is unsafe for consumption; it may just suggest that the water will have unpleasant aesthetic qualities in terms of color, taste, smell, etc. If you are concerned about the safety of your drinking water, you should have your water professionally tested.
Using Filter Paper and a Scale
Gather your materials. Prepare a clean, clear space with the appropriate instruments and tools for the task. If you do not have access to the materials necessary for this procedure, they can all be purchased easily online. You will need the following: A clean, properly sterilized beaker that is free of dust or other particles A sample of water, poured into the beaker Filter paper An evaporating dish A stirring stick A pipette large enough to collect a 50 ml sample A scale
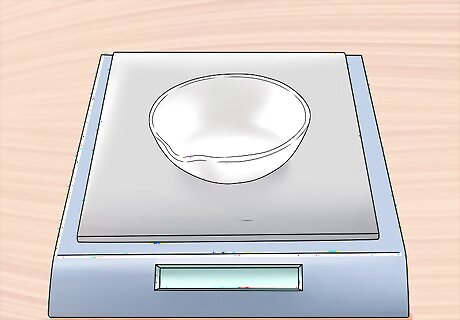
Weigh the evaporating dish in milligrams (mg). Make sure that it is completely dry and completely clean of any extraneous particulate matter.
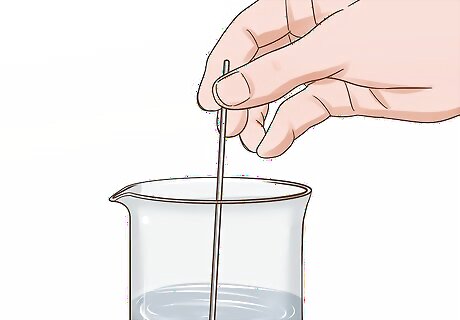
Stir the water sample in the beaker with your stirring stick. Stir vigorously enough to agitate the solution. This ensures that any particulate matter is more or less evenly distributed throughout the sample.
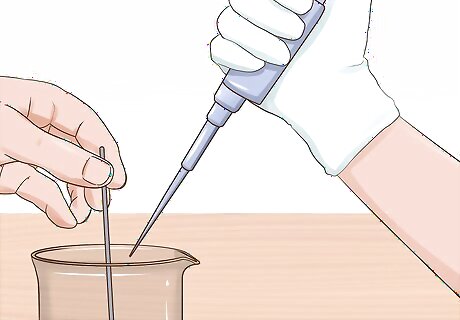
Collect 50 mL of the water in the pipette. Make sure you're still stirring the water while collecting the sample — don't let the solution settle before you pipette your smaller sample. If you find this difficult to accomplish, you might ask a friend to pipette the sample while you stir.
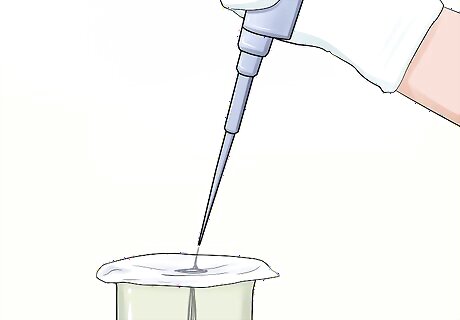
Extract the filtrate. Put the 50 mL water sample from the pipette through the filter paper three times to ensure all particulate matter has been collected in the filter.
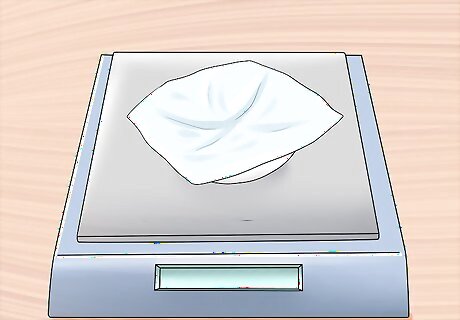
Weigh the evaporating dish with the filtrate. Transfer the filtrate from the previous step to the evaporating dish you weighed in step 2, and wait for the filtrate to dry completely. Once the dish and filtrate are dry, weigh them in milligrams (mg).
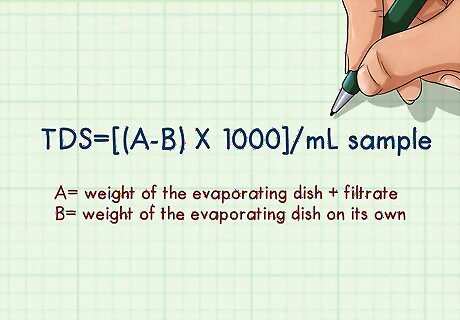
Plug your data into the formula. Use the following formula to calculate the TDS of your solution: TDS=[(A-B) * 1000]/mL sample In this formula, A stands for the weight of the evaporating dish + filtrate, and B stands for the weight of the evaporating dish on its own. Because you pipetted 50 mL of water, the value of "mL sample" in this case would be 50. The final value of the Total Dissolved Solids is measured in mg/L. Water with a TDS of less than 500 mg/L meets the Environmental Protection Agency's standards for drinking water. A high TDS does not necessarily mean that water is unsafe for consumption; it may just suggest that the water will have unpleasant aesthetic qualities in terms of color, taste, smell, etc. If you are concerned about the safety of your drinking water, you should have your water professionally tested.















Comments
0 comment