views
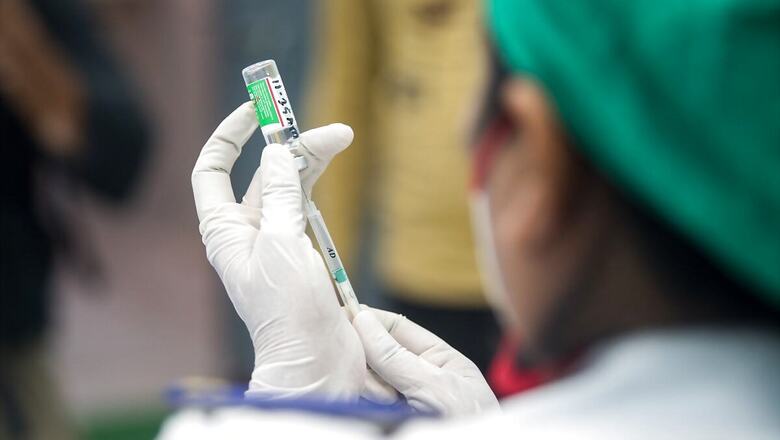
With the rollout of Covid-19 vaccines, people are increasingly asking which vaccine works best against the virus. After the second wave wreaked havoc across India, experts believe that vaccines are the best way forward for India, which launched its mass inoculation program in January. Since then, official data showed that only 11% of the population has received at least one vaccine dose while just 3.4% have completed the required two doses.
On June 19-20, Hyderabad-based biotechnology company Bharat Biotech, that manufactures Covaxin, submitted phase-III clinical trial data of its vaccine to the Drugs Controller General of India (DCGI). According to this data, Covaxin has shown 77.8% efficacy was approved by the Subject Expert Committee (SEC) under DCGI. Here’s a look at the efficacy of Covid-19 vaccines around the world:
Covaxin (77.8%):
Amid concerns over the effectiveness of Covaxin, Bharat Biotech submitted its Phase 3 trial data which said that the vaccine has shown 77.8% efficacy during the trials. The data is yet to be published in an internationally recognised, peer-reviewed journal. Bharat Biotech had earlier said the data will be published after it is submitted to the national regulator and will be released within approximately three months.
Covishield (63.09%):
Indian Council of Medical Research (ICMR) head Dr Balram Bhargava has last month said that the immunity after the first dose of Covishield was found to be strong and that the three months gap will give the best results. The vaccine 19 has an efficacy of 63.09% against symptomatic SARS-CoV-2 infection. While the vaccine has shown to work against new variants, more studies are being conducted to ascertain its efficacy in new variants.
The Russian COVID-19 vaccine Sputnik V, which was to be rolled out at Indraprastha Apollo and Madhukar Rainbow Children’s Hospital in Delhi, has been delayed for some days. Sputnik V uses two different viruses that cause the common cold (adenovirus) in humans. It employs a different vector for each of the two shots, given 21 days apart. According to Gamaleya and the RDIF, Sputnik V has demonstrated an efficacy rate of 92 per cent.
Pfizer BioNTech (88% after second dose)
: This is among the most sought-after vaccines globally and may be the second international vaccine after Sputnik V to be available in India. As per clinical trials, the Pfizer vaccine is 60-70% effective against variants of concern, “while antibodies produced may be lower than usual”. However, the vaccine was found to be only 33% effective against the variant found in India three weeks after the first dose. Two weeks after the second dose, the vaccine was found to be 88% effective at stopping symptomatic disease from the same variant.
Moderna (90%):
Moderna’s mRNA vaccine has over 90% efficacy and found to neutralise the Delta variant and elicit high antibody and immune response, across all age groups. The a vaccine requires two doses to be fully effective. It was found that up to 14 days after the first dose, the effectiveness was 50.8 percent. After that, it was about 92.1 percent.
Johnson & Johnson:
The Johnson & Johnson’s Janssen Covid-19 vaccine is currently not available in India. It is authorised by the US Food and Drug Administration for an emergency use authorization (EUA) which allows it to be distributed in the US for use in people aged 18 and above. According to a Times of India report, the US recommended a pause in giving the J&J vaccine while authorities examined six reports of the unusual clots, including a death, out of more than 6.8 million Americans given the one-dose vaccination so far. Its efficacy was tested in a clinical trial involving more than 40,000 people after which it was granted emergency use authorisation. The results from the clinical trial found that the vaccine was successful in protecting against mild to moderate and severe to critical cases of COVID-19.
Read all the Latest News, Breaking News and Coronavirus News here.













Comments
0 comment