views
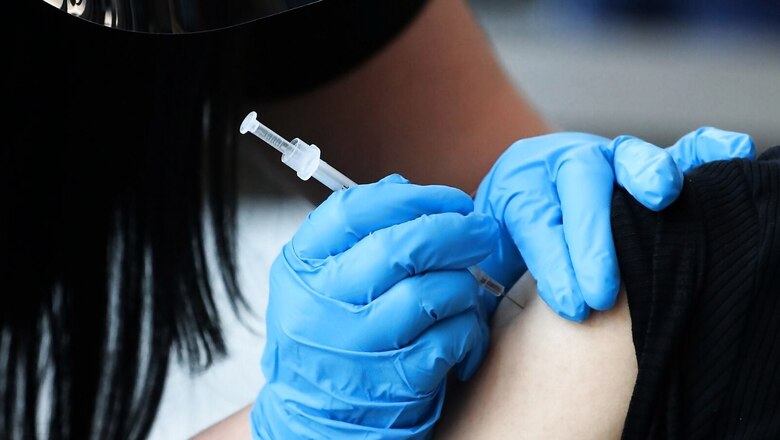
Indigenously developed Zydus Cadila Covid-19 vaccine ZyCoV-D on Friday received Emergency Use Authorisation from the Drug Controller General of India. The three-dose vaccine will be administered to people 12 years and above.
The announcement comes weeks after Johnson and Johnson‘s single-dose vaccine was cleared by the Indian regulator.
The DNA-based vaccine against the coronavirus joined a list of jabs to be available in India after Covishield, Covaxin, Sputnik V, Moderna and J&J.
Here is a list of all the vaccines approved in India so far, and how they work:
Covishield
The AstraZeneca-Oxford vaccine ‘Covishield’ is locally manufactured by the Serum Institute of India.
A weakened variant of a common cold virus (known as an adenovirus) from chimps is used to create the vaccine. It has been altered to resemble coronavirus, despite the fact that it cannot cause illness.
When a patient receives the vaccination, it triggers the immune system to produce antibodies and prepares it to fight any coronavirus infection.
The vaccination is given in two doses, four to twelve weeks apart. It can be safely maintained at temperatures ranging from 2 to 8 degrees Celsius and simply supplied in existing health-care facilities such as doctors’ offices.
Delayed Doses Work Better: Study
Delayed second and third doses of the AstraZeneca vaccine boost immunity against Covid-19, a study by Oxford University, which developed the jab with the British-Swedish firm, said recently.
An interval of up to 45 weeks between the first and second dose of the AstraZeneca vaccine led to an enhanced immune response, rather than compromising immunity, the study said.
Giving a third dose of the jab more than six months after the second dose also leads to a “substantial increase” in antibodies and induces a “strong boost” to subjects’ immune response, said the pre-print study, meaning that it has yet to be peer-reviewed.
“This should come as reassuring news to countries with lower supplies of the vaccine, who may be concerned about delays in providing second doses to their populations,” said lead investigator of the Oxford trial, Andrew Pollard.
Covaxin
Covaxin is an inactivated vaccine, meaning it is made up of coronaviruses that have been killed, making it safe to inject into the body.
Bharat Biotech, a 24-year-old vaccine company that exports to 123 countries and has a portfolio of 16 vaccines, using a coronavirus sample isolated by India’s National Institute of Virology.
Immune cells can still recognise the dead virus after it has been injected, prompting the immune system to produce antibodies against the pandemic virus.
The two dosages are separated by 4 weeks. The vaccination can be kept at temperatures ranging from 2 to 8 degrees Celsius.
Sputnik V
The vaccine, created by the Gamaleya Institute in Moscow, caused some controversy when it was first rolled out before the final study results were disclosed. However, scientists claim that its advantages have now been shown.
It employs a harmless cold-type virus as a carrier to deliver a small portion of the coronavirus to the body. The body begins to manufacture antibodies that are specifically adapted to the virus after being vaccinated.
It may be held at temperatures ranging from 2 to 8 degrees Celsius (a conventional fridge is around 3-5 degrees Celsius), making it more convenient to transport and store.
The Sputnik vaccine, unlike other similar vaccinations, employs two slightly different forms of the vaccine for the first and second doses, which are given 21 days apart.
They both aim for the coronavirus’s unique “spike,” but they use different vectors – neutralised viruses that carry the spike to the body.
The theory is that utilising two distinct formulae strengthens the immune system even more than using the same one twice, and may provide longer-term protection.
Moderna
Moderna’s method to protect against Covid-19 relies on messenger RNA (mRNA) to program cells to generate immunity to the coronavirus. This vaccine along with Pfizer are viewed as a preferred choice among wealthy countries, analysts said, based on clinical trial data showing they were more than 90% effective at preventing symptomatic coronavirus.
About 120 million Americans have received a Pfizer or Moderna shot so far with no major safety issue identified.
The United States and European Union are pushing to stock up on even more of the mRNA vaccines. Japan is also working to secure 100 million doses of Pfizer’s shot by the end of June.
The higher cost, production limits and demanding requirements for shipping and storage could limit mRNA-based vaccines’ availability in lower income countries, experts said.
Johnson & Johnson
Johnson & Johnson’s single-dose COVID-19 vaccine has been given Emergency Use approval in India, Union Health minister Mansukh Mandaviya said. He said this will further boost the country’s collective fight against the novel coronavirus infection.
“India expands its vaccine basket! Johnson and Johnson’s single-dose COVID-19 vaccine is given approval for Emergency Use in India. Now India has five EUA vaccines. This will further boost our nation’s collective fight against #COVID19,” the minister tweeted. The US-based pharmaceutical company had applied for Emergency Use Authorisation for its jab on Friday and was granted the approval the same day by the Drugs Controller General of India (DCGI), a senior official said.
J&J earlier had sought approval to conduct phase-3 clinical trial of its vaccine on approximately 600 participants in two age groups—those aged 18 and below 60 years and those aged 60 years and above—to evaluate the safety, reactogenicity and immunogenicity of the jab in healthy Indian adults.
However, on July 29, the firm withdrew its proposal. A health ministry official at a recent press conference had clarified that J&J earlier applied for conducting phase-3 clinical trial of its vaccine.
Zydus Cadila
Indian made Zydus Cadila Covid vaccine ZyCoV-D has received approval for Emergency Use Authorisation from the DCGI and it will be administered to people 12 years and above, the Department of Biotechnology (DBT) said on Friday.
“This is the world’s first DNA-based vaccine against the coronavirus, and this three-dose vaccine when injected produces the spike protein of the SARS-CoV-2 virus and elicits an immune response, which plays a vital role in protection from the disease as well as viral clearance,” the statement said.
The DBT said the “plug-and-play” technology on which the plasmid DNA platform is based can be easily adapted to deal with mutations in the virus, such as those already occurring. The vaccine has been developed in partnership with the DBT under Mission COVID Suraksha.
“It has been implemented by the BIRAC (Biotechnology Industry Research Assistance Council) and ZyCoV-D has been supported under the COVID-19 Research Consortia through National Biopharma Mission for preclinical studies, Phase I and Phase II clinical trials and under the Mission COVID Suraksha for Phase III Clinical Trial,” it added.
Expected Vaccines
Corbevax
The Centre has placed an advance order for 30 crore doses of a vaccine being created by the Hyderabad-based Biological-E called Corbevax. Not only is it an indigenous vaccine, it also could potentially be the cheapest vaccine available in India when it finally launches. So, what makes Corbevax tick?
HOW SOON WILL IT BE AVAILABLE?
The vaccine has received the nod for Phase III clinical trials in India after showing promising results in phases I and II. The Centre will pay Rs 1,500 crore for its consignment of 30 crore doses. Corbevax, like most other Covid-19 jabs out so far, is a two-dose vaccine.
After the completion of the Phase III trials, production to meet the Centre’s delivery target will continue between August and December.
The vaccine is reportedly going to be the cheapest vaccine available in India with the two shots expected to be cumulatively priced below Rs 400. In comparison, the Covishield vaccine which come at Rs 300-Rs 400 for a single dose. The Russian Sputik V, the third vaccine that has received emergency approval for use in India, costs around Rs 1,000.
Novovax
The manufacturing of the first batch of Covovax vaccine has started at the Pune facility of the Serum Institute of India, the firm announced recently.
“A new milestone has been reached; this week we began our first batch of Covovax (a COVID-19 vaccine developed by Novavax) at our facility, here in Pune: Serum Institute India,” the company shared on Twitter.
The SII is hoping to launch Novavax’s COVID-19 vaccine ‘Covovax’ in India by September as its trials are in an advanced stage of completion, its CEO Adar Poonawalla had earlier said.
In September 2020, Novavax announced a manufacturing agreement with SII for its COVID-19 vaccine NVX-CoV2373. CNBC-TV18 reported according to Poonawalla, the trial of Novavax’s coronavirus vaccine in India is likely to conclude by November. The pharmaceutical giant can apply for a vaccine license even before its trial in the country concludes on the basis of the global data of the trial, he had added.
Novavax in a statement on June 14 had stated that the vaccine candidate ‘NVX-CoV2373’ demonstrated 100 percent protection against moderate and severe Covid-19 infection- 90.4 percent efficacy overall. And it also met the primary endpoint in its PREVENT-19 pivotal Phase 3 trial.
Read all the Latest News, Breaking News and Assembly Elections Live Updates here.















Comments
0 comment